Abstract
PLX51107 is a novel BET inhibitor (BETi) with antineoplastic activity in pre-clinical models of both aggressive B-cell malignancies and AML. Differentiating it from other BET inhibitors currently under investigation, PLX51107 has a unique binding site in the acetylated lysine binding pocket of BRD4. Through interaction with pTEFb, which is comprised of CDK9 and several cyclin T1 heterodimers, BRD4 promotes phosphorylation of RNA polymerase II, ultimately influencing gene transcription. Dinaciclib is a potent inhibitor of multiple cyclin dependent kinases including CDK9 and has been shown to have pre-clinical activity in AML. Therefore, we hypothesized that PLX51107 would have synergistic activity when combined with dinaciclib.
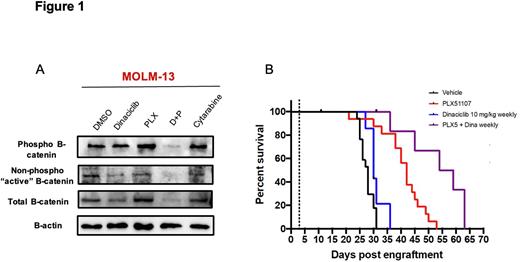
We found synergistic effects on proliferation in primary AML cells when PLX51107 was combined with dinaciclib in stromal co-cultures across a range of dose combinations below the IC50 of either compound alone. Synergistic activity was also present in cytokine support culture conditions, colony forming assays, and synergy assays utilizing COMbenefit software. Interestingly, the highly conserved canonical β-catenin dependent Wnt signaling pathway is a known BETi resistance mechanism in AML. Therefore, destruction of β-catenin and inhibition of Wnt signaling could represent a novel therapeutic target to circumvent development of BET-mediated resistance. We found dinaciclib treatment reduced protein levels of total β-catenin in primary AML cells and AML cell lines (Figure 1A). Phosphorylated β-catenin and non-phosphorylated "active" β-catenin protein levels were also reduced following dinaciclib treatment (Figure 1A). In addition, mRNA expression of multiple components of the Wnt pathway, including the Wnt co-receptor LRP6, AXIN2, FZD1, and many Wnt target genes were significantly decreased following dinaciclib and combination treatment. Moreover, in a luciferase based Wnt reporter cell line, we found dinaciclib significantly reduced Wnt3a induced luciferase activity, suggesting a direct effect on β-catenin mediated transcription of Wnt target genes. Collectively, this data suggests dinaciclib has multiple inhibitory effects on canonical β-catenin dependent Wnt signaling.
We found combination treatment of PLX51107 and dinaciclib produced synergistic reduction in expression of both Wnt signaling components and Wnt target genes. Conversely, β-catenin and Wnt target gene expression levels were either not significantly changed or even increased when treated with PLX51107 alone. In order to examine the effects of dinaciclib in the setting of BET resistance, we developed PLX51107 resistant MOLM-13 and MV4-11 AML cell lines via serial drugging. These resistant cell lines remained sensitive to dinaciclib treatment and to cytotoxic chemotherapy drugs including cytarabine and etoposide while demonstrating resistance to PLX51107 via Annexin PI and MTS assays. We found sensitivity to dinaciclib treatment in these resistant cell lines remained unaffected by BETi resistance and continued to demonstrate inhibition of Wnt signaling, similar to the effects seen in parental cells. Therefore, dinaciclib represents a novel strategy to overcome potential therapy emergent BETi resistance.
We next examined the efficacy of PLX51107 in combination with dinaciclib in vivo, utilizing luciferase labeled MOLM-13 AML cells xenotransplanted into NOD / SCID / IL2rgnull (NSG) immunodeficient mice. Daily oral dosing with 20 mg/kg PLX51107 resulted in prolonged survival (median 42 days) compared to vehicle treated control animals (median 28 days, p< 0.001). Weekly intraperitoneal 10 mg/kg dinaciclib did not significantly improve survival (median 30 days, p=0.09) in MOLM-13 xenograft mice. However, mice receiving combination therapy survived a median of 56.5 days, significantly longer than with either drug alone (p<0.05) (Figure 1B). We also found decreased disease burden by bioluminescence and by histopathology in combination treated animals, compared to both vehicle and single agent controls. In conclusion, we have demonstrated in vitro and in vivo synergistic activity of the BET inhibitor PLX51107 in combination with dinaciclib in pre-clinical models of AML and have identified novel inhibition of Wnt / β-catenin signaling by dinaciclib, thereby providing a strategy to circumvent resistance to BET inhibitors in AML.
Disclosures
Mims:Jazz Pharmaceuticals: Other: Data Safety and Monitoring Board; AbbVie: Membership on an entity's Board of Directors or advisory committees; Ryvu: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Zentalis: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Other: Data Safety and Monitoring Board; Genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Larkin:Gilead: Membership on an entity's Board of Directors or advisory committees. Vasu:Boehringer Ingelheim: Other: Travel support; Seattle Genetics: Other: Travel support. Blachly:AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; MingSight Pharmaceuticals: Research Funding; INNATE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees. Byrd:Janssen Pharmaceuticals, Inc.: Consultancy; Novartis: Consultancy, Honoraria; Kura Oncology, Inc: Consultancy; Pharmacyclics LLC: Honoraria, Research Funding; TG Therapeutics: Honoraria; AstraZeneca: Consultancy; Xencor, Inc: Research Funding; Vincerx Pharma: Current equity holder in publicly-traded company; Syndax: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal